Publications
2026
- ARXIV
 Learning and Naming Subgroups with Exceptional Survival CharacteristicsMhd Jawad Al Rahwanji, Sascha Xu, Nils Philipp Walter, and Jilles VreekenFeb 2026arXiv:2602.22179
Learning and Naming Subgroups with Exceptional Survival CharacteristicsMhd Jawad Al Rahwanji, Sascha Xu, Nils Philipp Walter, and Jilles VreekenFeb 2026arXiv:2602.22179In many applications, it is important to identify subpopulations that survive longer or shorter than the rest of the population. In medicine, for example, it allows determining which patients benefit from treatment, and in predictive maintenance, which components are more likely to fail. Existing methods for discovering subgroups with exceptional survival characteristics require restrictive assumptions about the survival model (e.g. proportional hazards), pre-discretized features, and, as they compare average statistics, tend to overlook individual deviations. In this paper, we propose Sysurv, a fully differentiable, non-parametric method that leverages random survival forests to learn individual survival curves, automatically learns conditions and how to combine these into inherently interpretable rules, so as to select subgroups with exceptional survival characteristics. Empirical evaluation on a wide range of datasets and settings, including a case study on cancer data, shows that Sysurv reveals insightful and actionable survival subgroups.
@misc{Al_Rahwanji_2026, title = {Learning and Naming Subgroups with Exceptional Survival Characteristics}, author = {Al Rahwanji, Mhd Jawad and Xu, Sascha and Walter, Nils Philipp and Vreeken, Jilles}, journal = {arXiv}, note = {arXiv:2602.22179}, year = {2026}, month = feb, eprint = {2602.22179}, archiveprefix = {arXiv}, primaryclass = {cs.LG}, url = {https://arxiv.org/abs/2602.22179}, }
2023
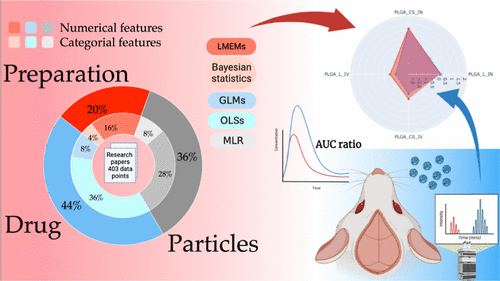
- A comprehensive study on nanoparticle drug delivery to the brain: application of machine learning techniquesAmal Yousfan, Mhd Jawad Al Rahwanji, Abdulsamie Hanano, and Hisham Al-ObaidiMolecular Pharmaceutics, Dec 2023PMID: 38060692
The delivery of drugs to specific target tissues and cells in the brain poses a significant challenge in brain therapeutics, primarily due to limited understanding of how nanoparticle (NP) properties influence drug biodistribution and off-target organ accumulation. This study addresses the limitations of previous research by using various predictive models based on collection of large data sets of 403 data points incorporating both numerical and categorical features. Machine learning techniques and comprehensive literature data analysis were used to develop models for predicting NP delivery to the brain. Furthermore, the physicochemical properties of loaded drugs and NPs were analyzed through a systematic analysis of pharmacodynamic parameters such as plasma area under the curve. The analysis employed various linear models, with a particular emphasis on linear mixed-effect models (LMEMs) that demonstrated exceptional accuracy. The model was validated via the preparation and administration of two distinct NP formulations via the intranasal and intravenous routes. Among the various modeling approaches, LMEMs exhibited superior performance in capturing underlying patterns. Factors such as the release rate and molecular weight had a negative impact on brain targeting. The model also suggests a slightly positive impact on brain targeting when the drug is a P-glycoprotein substrate.
@article{Yousfan_2023, author = {Yousfan, Amal and Al Rahwanji, Mhd Jawad and Hanano, Abdulsamie and Al-Obaidi, Hisham}, title = {A comprehensive study on nanoparticle drug delivery to the brain: application of machine learning techniques}, journal = {Molecular Pharmaceutics}, year = {2023}, month = dec, publisher = {American Chemical Society}, doi = {10.1021/acs.molpharmaceut.3c00880}, note = {PMID: 38060692}, url = {https://doi.org/10.1021/acs.molpharmaceut.3c00880}, } - Seroprevalence and trends of hepatitis B virus, hepatitis C virus and human immunodeficiency virus in Syrian blood donors at Damascus University Blood Center between 2004 and 2021Alia Alassad, Mhd Jawad Al Rahwanji, Amal Yousfan, Sally Al Moualem , and 2 more authorsFrontiers in Public Health, Jun 2023
Introduction: Seroprevalence of transfusion-transmitted viral infections (TTVIs) is a valuable indicator for assessing blood safety, population health and health system performance in the times of peace and conflicts. Only scarce information is available on the impact of the decade-long violent conflict on the prevalence of TTVIs in Syria. Moreover, hepatitis B vaccine was introduced to the national vaccination program in 1993; however, no data is available on the vaccine effectiveness. Methods: In this retrospective cross-sectional study, we compiled the screening results for major TTVIs, namely hepatitis B virus (HBV), hepatitis C virus (HCV), and human immuno-deficiency virus (HIV), of volunteer donors at Damascus University Blood Center from May 2004 to October 2021. Prevalence was expressed in percentages for the entire study group and subgroups. Chi-square test and linear regression were used to examine the differences and describe trends in prevalence, respectively, based on demographic characteristics (i.e., age and gender) and time. P-value of 0.005 was considered statistically significant. Results: Of the total 307,774 donors (82.27% males, median age 27 years), 5,929 (1.93%) had serological evidence of at least one TTVI, and 26 (0.0085%) had multiple infections. The lowest prevalence (1.09%) was detected in donors aged 18–25 years old, and a higher prevalence (2.05%) was evident in males in comparison with females (1.38%). The seroprevalence of HBV, HCV, and HIV was 1.18, 0.52, and 0.23%, respectively. Trend analyses revealed a significant regression in HBV and HIV prevalence from 2011 to 2021. HBV seropositivity depicted a temporal decline by 80%, from 0.79% in 2011 to 0.16% in 2021 in those born in 1993 and thereafter. Discussion: The seroprevalence of HBV, HIV, and to a lesser extent HCV dropped over the study 18-year period. Possible explanations may include implementation of the HBV vaccine, robust national health system, conservative sociocultural values, and isolation.
@article{Alassad_2023, author = {Alassad, Alia and Al Rahwanji, Mhd Jawad and Yousfan, Amal and Al Moualem, Sally and Farhat, Arwa and Youssef, Lama A.}, title = {Seroprevalence and trends of hepatitis B virus, hepatitis C virus and human immunodeficiency virus in Syrian blood donors at Damascus University Blood Center between 2004 and 2021}, journal = {Frontiers in Public Health}, volume = {11}, year = {2023}, month = jun, url = {https://www.frontiersin.org/articles/10.3389/fpubh.2023.1174638}, doi = {10.3389/fpubh.2023.1174638}, issn = {2296-2565}, }
2022
- The optimal period for oocyte retrieval after the administration of recombinant human chorionic gonadotropin in in vitro fertilizationMhd Jawad Al Rahwanji, Homam Abouras, Mhd Said Shammout, Ray Altalla , and 3 more authorsBMC Pregnancy and Childbirth, Mar 2022
Background: Our objective was to investigate the existence of an optimal period for oocyte retrieval in regards to the clinical pregnancy occurrence after the administration of recombinant human chorionic gonadotropin (rhCG) (Ovitrelle®). Methods: We studied the digital records of 3362 middle eastern couples who underwent in vitro fertilization (IVF) treatment between 2019 and 2021. Results: Through statistical testing, we found that there is a significant positive correlation between the oocyte retrieval period and the clinical pregnancy occurrence up to the 37th hour, where retrieval at the 37th hour was found to provide the most optimal outcome, especially in the case of gonadotropin-releasing hormone agonist (GnRHa) long protocol. Conclusions: This cohort study recommends retrieval at hour 37 after ovulation triggering under the described conditions.
@article{Al_Rahwanji_2022, title = {The optimal period for oocyte retrieval after the administration of recombinant human chorionic gonadotropin in in vitro fertilization}, author = {Al Rahwanji, Mhd Jawad and Abouras, Homam and Shammout, Mhd Said and Altalla, Ray and Al Sakaan, Reem and Alhalabi, Nawras and Alhalabi, Marwan}, journal = {BMC Pregnancy and Childbirth}, volume = {22}, number = {1}, year = {2022}, month = mar, publisher = {Springer Science and Business Media {LLC}}, issn = {1471-2393}, url = {https://doi.org/10.1186/s12884-022-04412-9}, doi = {10.1186/s12884-022-04412-9}, }